Cytology of Mast Cell Tumors in Veterinary Medicine: A Clinical Perspective
Mast cell tumors (MCTs) represent one of the most diagnostically rewarding yet prognostically challenging neoplasms in veterinary medicine. In dogs, they account for approximately 20% of all cutaneous neoplasms, with roughly 10% demonstrating malignant behavior that can range from easily curable with surgical excision to aggressive tumors with poor prognosis and survival times under four months.¹'² The stark contrast in biological behavior within this single tumor type has driven the evolution of increasingly sophisticated diagnostic approaches that can predict clinical outcomes directly from cytological samples.
Fine needle aspiration cytology has emerged as the cornerstone of MCT diagnosis, with diagnostic accuracy heavily dependent on sample quality, staining methodology, and cellular preservation. When optimal techniques are employed, including appropriate needle selection, adequate sampling, and Wright-Giemsa staining, diagnostic accuracy can approach 95%.³'⁴ However, this high accuracy rate assumes proper sample preparation and excludes poorly cellular or heavily blood-contaminated samples that may compromise diagnostic reliability. Additionally, the unprecedented advantage of prognostic assessment through cytological grading requires even more stringent sample quality criteria, with at least 100 intact mast cells needed for reliable grading assessment.⁵ This represents a potential paradigm shift from traditional approaches that required histopathological examination for grading, though successful implementation depends critically on technical expertise and quality control measures.
The development of cytological grading systems has transformed clinical decision-making for MCTs, bridging the critical gap between diagnosis and prognosis.⁵ However, the practical implementation of these systems in clinical practice requires understanding not only the technical aspects but also the clinical context that determines when and how these tools should be applied.
Historical Context and Evolution of Understanding
The journey of mast cell tumor understanding began with Paul Ehrlich's initial description in 1877, when he named these cells "Mastzellen" based on the mistaken belief that their granules served a nutritional function. The German word "Mast" denoting "fattening" reflected his observation that these cells were abundant in chronic inflammation and tumors, which he attributed to the nutritional requirements of these tissues. The revolutionary understanding of mast cell function emerged between 1952-1956, when research into histamine, anaphylactic responses, and heparin coalesced to reveal that the dark granules actually contained histamine and other powerful inflammatory mediators. This discovery fundamentally changed our perception of mast cells from nutritional support cells to key players in immune and inflammatory responses. Modern understanding recognizes mast cells as sophisticated cellular factories producing numerous bioactive compounds including histamine for vascular and glandular responses, eicosanoids for microvascular permeability and bronchoconstriction, proteases and proteoglycans for antimicrobial defense and tissue remodeling, and various cytokines and growth factors that orchestrate inflammatory responses.⁶ These cells also contribute to angiogenesis, immune tolerance, and wound healing, making their neoplastic transformation particularly complex from both biological and clinical perspectives.
Species-Specific Considerations in Clinical Practice
Canine Mast Cell Tumors: The Clinical Challenge
Canine MCTs present the greatest diagnostic and prognostic complexity in veterinary oncology, with their wide range of biological behavior creating significant clinical decision-making challenges. The approximately 10% malignancy rate among cutaneous MCTs masks the reality that predicting which tumors will behave aggressively remains one of the most critical challenges in veterinary oncology.⁷'⁸
Location plays a crucial role in prognosis, with preputial, scrotal, digital, perioral, and muzzle locations associated with worse outcomes regardless of cytological or histological grade.⁹'¹⁰ This anatomical consideration must be integrated with cytological findings to provide comprehensive prognostic assessment for clinical decision-making.
The distinction between cutaneous and subcutaneous MCTs has profound clinical implications that directly impact treatment planning. Subcutaneous MCTs demonstrate remarkably favorable behavior, with more than 90% controlled by surgical excision alone and overall recurrence rates less than 10%.¹¹ Complete excision reduces recurrence to only 2%, while incomplete excision results in 12% recurrence rates. This dramatic difference in biological behavior makes accurate anatomical localization essential for appropriate treatment planning.
Feline Mast Cell Tumors: A Different Clinical Entity
Feline MCTs represent a fundamentally different clinical challenge compared to their canine counterparts, accounting for approximately 21% of all cutaneous neoplasms but demonstrating predominantly benign behavior.¹² The majority of cutaneous feline MCTs behave benignly and can be cured with surgical excision alone, making aggressive treatment protocols typically unnecessary.
However, approximately 10% of feline cutaneous MCTs demonstrate aggressive behavior, characterized by multiple tumors, spread to local lymph nodes, low to moderate cytoplasmic granularity, high Ki67 index, and mitotic counts exceeding 5 per 10 high-power fields.¹³ The identification of these aggressive cases remains challenging, as established canine grading systems may not directly apply to feline tumors.
Feline visceral MCTs carry uniformly poor prognosis with widespread metastasis and dissemination common, though no established grading system exists for cats.¹⁴ The unique biological behavior of feline MCTs necessitates species-specific diagnostic approaches and treatment protocols.
Cytological Grading: From Research to Clinical Implementation
The Clinical Reality of Grading Systems
The development of cytological grading systems represents one of the most significant advances in veterinary oncology, yet their clinical implementation requires understanding both their capabilities and limitations. The transition from the traditional Patnaik three-tier system to modern two-tier approaches reflects the practical reality that intermediate-grade tumors created prognostic uncertainty rather than clarity.
The Patnaik grading system, introduced in 1984, demonstrated significant limitations in clinical application with Grade 2 MCTs accounting for up to 70% of cases in some studies, creating a prognostic "gray zone" that hindered treatment planning.¹⁵'¹⁶ Inter-observer agreement proved problematic, with studies reporting only 62-63% concordance for Grade I and II tumors, though Grade III tumors achieved 74% concordance.¹⁷'¹⁸
The two-tier system addressed these limitations by providing clearer prognostic categories with better inter-observer reliability. This approach reclassified all former Grade I tumors as low grade and all Grade III tumors as high grade, while dividing Grade II tumors into 84% low grade and 16% high grade based on specific morphological criteria.¹⁹ The prognostic separation proved dramatic, with low-grade tumors showing 2-year median survival times compared to 4-month median survival for high-grade tumors.
The Camus Cytological Grading System: Clinical Validation
The landmark study by Camus et al. provided the first cytological grading system with robust clinical validation, incorporating patient outcomes rather than simply comparing cytological and histological findings.⁵ This approach addressed the fundamental clinical question of whether cytological assessment could reliably predict patient outcomes without requiring tissue biopsy.
The system classifies tumors as high grade based on poor granulation (poorly granulated cells predominate) or the presence of at least two of four additional criteria: mitotic figures present, binucleated or multinucleated cells present, nuclear pleomorphism present, or greater than 50% anisokaryosis.⁵ This straightforward approach enables rapid assessment while maintaining prognostic accuracy.
Clinical validation demonstrated that dogs with cytologically high-grade MCTs were 25 times more likely to die within 2 years compared to those with low-grade tumors.⁵ However, the system showed 31.8% false positives for high grade and 1.6% false negatives, with overall agreement between cytological and histological grading of 73-77%.⁵ In practical terms, this means that while the grading system effectively identifies truly aggressive tumors, approximately one-third of tumors identified as high-grade cytologically may actually behave more favorably than predicted, while a small percentage of genuinely aggressive tumors may be misclassified as low-grade. These statistics highlight both the utility and limitations of cytological grading in clinical practice.
Practical Implementation Considerations
The question of whether to implement cytological grading in clinical practice requires balancing the benefits of immediate prognostic information against the technical requirements and potential limitations.²⁰ The exclusion criteria requiring at least 100 intact mast cells for reliable grading assessment means that not all diagnostic samples will be suitable for prognostic evaluation.
Sample quality considerations extend beyond cell counts to include adequate cellular preservation and minimal blood contamination.²¹ The choice of staining method can significantly impact both diagnostic accuracy and grading reliability, with Wright-Giemsa staining preferred over rapid staining methods for detailed morphological assessment required for grading.²²
The integration of cytological grading into clinical workflow requires establishment of quality assurance protocols, including regular correlation with histopathological findings and clinical outcomes.²⁰ Training requirements for accurate implementation may necessitate specialized cytopathology expertise or referral to experienced laboratories.
Morphological Assessment: The Foundation of Cytological Diagnosis
Granulation Patterns: The Key Prognostic Feature
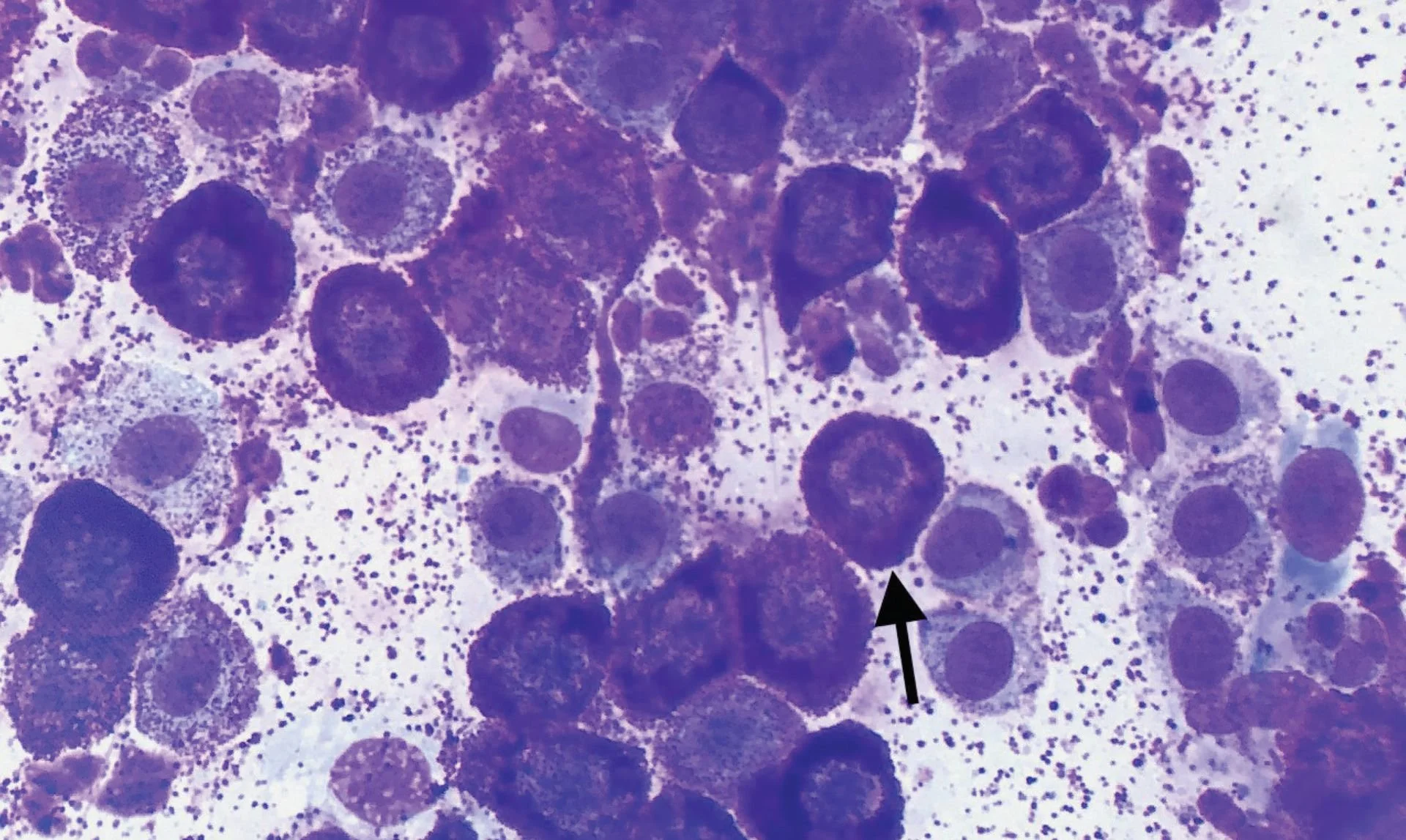
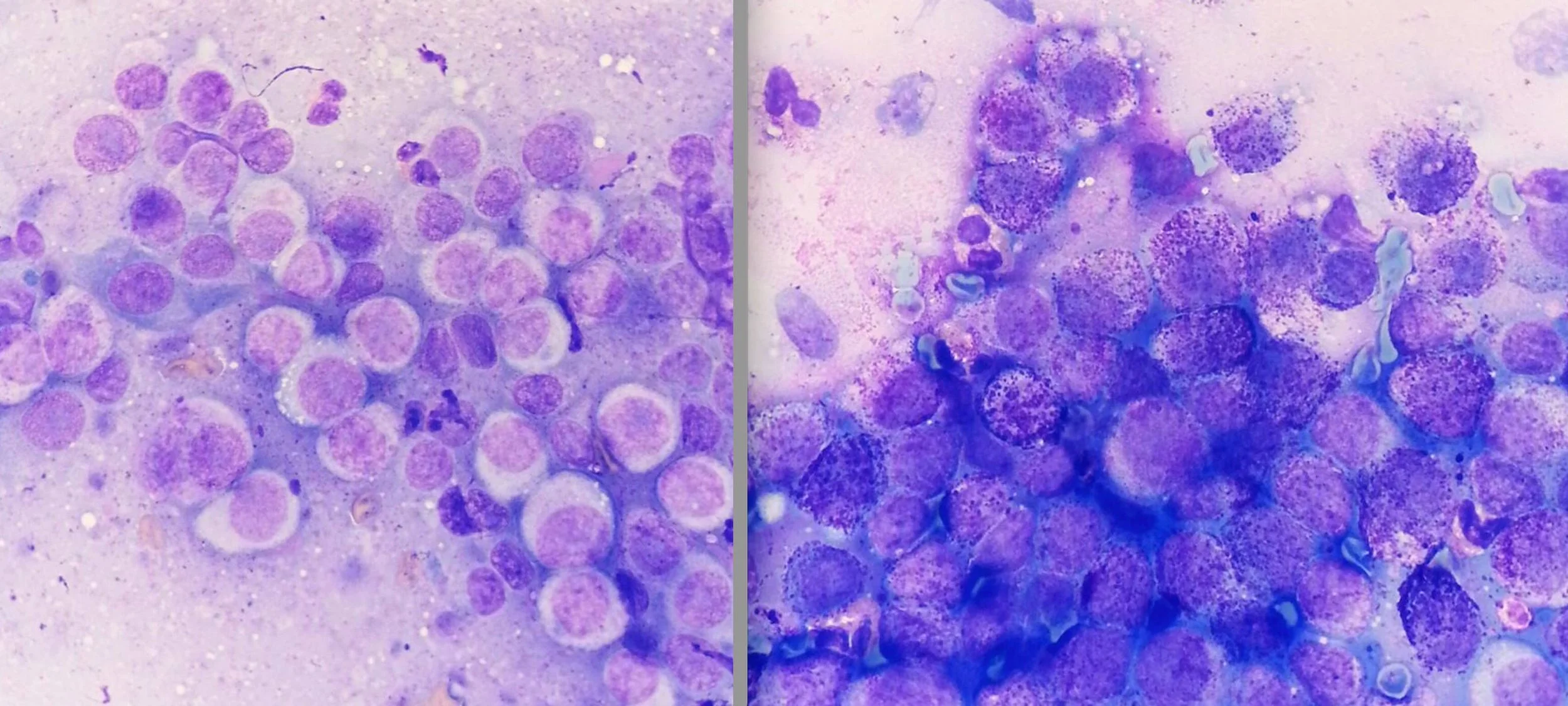
Granulation assessment represents the most prognostically significant parameter in cytological grading, demonstrating the strongest correlation with 2-year survival outcomes (R² = 0.40, P < 0.001).⁵ The evaluation requires systematic assessment of the entire cellular population to determine the predominant granulation pattern, as focal areas of altered granulation may not reflect overall tumor behavior.
Well-granulated mast cells contain abundant, distinct cytoplasmic granules that may partially obscure nuclear detail but generally indicate favorable prognosis. The dense granulation often makes nuclear assessment challenging, requiring careful evaluation to identify nuclear features that contribute to grading assessment.
Poorly granulated cells present with few to absent cytoplasmic granules, allowing clear visualization of nuclear morphology but signaling more aggressive biological behavior.²³ The absence of granulation can create diagnostic challenges, as poorly granulated MCTs may be confused with other round cell tumors, particularly lymphoma or histiocytic neoplasms.
Mixed granulation patterns require careful assessment to determine the predominant cellular population, as the prognostic significance depends on which population predominates rather than the mere presence of poorly granulated cells. Quantitative assessment approaches may help standardize this evaluation and improve inter-observer reliability.²⁴
Nuclear Morphological Features: Beyond Granulation
Nuclear characteristics provide crucial supplementary information that enhances prognostic assessment beyond granulation patterns alone. Anisokaryosis, defined as greater than 50% variation in nuclear size, demonstrates a correlation coefficient of R² = 0.27 with P < 0.001 for survival outcomes.⁵ This parameter requires objective assessment that may benefit from morphometric approaches to ensure consistency across observers and institutions.
Nuclear pleomorphism encompasses evaluation of nuclear shape irregularities and contour abnormalities that indicate malignant transformation. The assessment requires recognition of subtle changes in nuclear architecture, including membrane irregularities, nuclear indentations, and bizarre nuclear shapes that suggest genetic instability.²⁵
Multinucleated cells, including both binucleate and multinucleated variants, indicate defective cellular division processes and correlate with aggressive behavior (R² = 0.21, P < 0.001).⁵ However, recent research has questioned whether binucleation should be included as a grading criterion, as some studies suggest it may not significantly contribute to prognostic accuracy.²⁶
Mitotic activity assessment in cytological preparations requires careful examination at high magnification, though mitotic figures occur less frequently in cytological samples compared to histological sections. The presence of identifiable mitotic figures in cytological preparations carries significant prognostic weight (R² = 0.16, P < 0.001) despite their reduced frequency.⁵
Clinical Staging and Treatment Integration
The Reality of Clinical Staging
Clinical staging remains essential for all MCTs regardless of grade, as any mast cell tumor is capable of metastasis.²⁷ The dramatic difference in survival times between stages emphasizes the critical importance of comprehensive staging protocols. Stage 1 tumors demonstrate median survival of 6.2 years, while Stage 2-3 tumors with lymph node involvement show median survival of only 0.8 years.²⁸
Lymph node assessment requires systematic cytological evaluation using established classification schemes for interpreting mast cell aggregates.²⁹ The presence of 2-3 aggregates of 2-3 mast cells indicates possible metastasis, while more than 3 aggregates of 2-3 mast cells or 2-5 aggregates of more than 3 mast cells suggests probable metastasis. Large numbers of aggregates or poorly differentiated mast cells indicate certain metastasis.
The discrepancy between histological and cytological evaluation of lymph nodes in 20% of cases highlights the importance of correlation between assessment methods and the potential need for histopathological confirmation in cases with discordant findings.³⁰
Treatment Planning Based on Cytological Assessment
Low-grade MCTs identified through cytological assessment can typically be managed with surgical excision alone, provided adequate margins can be achieved.³¹ This conservative approach allows for excellent outcomes while avoiding unnecessary aggressive treatment protocols that may increase morbidity and cost.
High-grade MCTs require immediate implementation of comprehensive staging protocols and consideration of aggressive treatment approaches.³² The identification of high-grade characteristics triggers extensive diagnostic workups and may necessitate referral to veterinary oncology specialists for optimal management.
Subcutaneous MCTs represent a special category where the overwhelmingly benign behavior supports conservative surgical management in most cases.¹¹ However, specific criteria including mitotic counts exceeding 4 per 10 high-power fields, Ki67 index above 21.8%, and other ancillary diagnostic findings may identify the small subset with malignant potential.
Ancillary Diagnostic Methods: Enhancing Prognostic Assessment
Proliferation Markers in Clinical Context
Ki67 immunohistochemistry provides quantitative assessment of cellular proliferation that complements morphological grading criteria.³³ This protein associated with cell proliferation and ribosomal RNA transcription offers objective measurement of tumor growth potential that may enhance prognostic accuracy when integrated with cytological findings.
AgNOR (argyrophilic nucleolar organizer regions) assessment quantifies silver-loving proteins in nucleolar areas that correlate with cell duplication rates. Malignant cells frequently demonstrate greater AgNOR counts, and the AgNOR×Ki67 score above 55 has been identified as a prognostic indicator for subcutaneous MCTs.³⁴
The integration of proliferation markers with cytological grading may provide enhanced prognostic stratification, particularly for cases with borderline morphological features or discordant clinical presentations.³⁵
KIT Expression and Mutation Analysis
KIT (CD117) represents a crucial diagnostic and prognostic marker for MCTs, as this tyrosine kinase receptor is expressed by virtually all mast cells and maintains expression throughout maturation, unlike most other cell types where expression decreases with maturation.³⁶
The evaluation of KIT localization patterns provides additional prognostic information, with Pattern 1 (membrane localization), Pattern 2 (focal cytoplasmic staining), and Pattern 3 (diffuse cytoplasmic staining) potentially correlating with biological behavior, though study results remain somewhat discordant regarding the prognostic significance of these patterns.³⁷'³⁸
KIT mutation analysis, particularly screening for mutations in exons 8 and 11, may help predict efficacy of tyrosine kinase inhibitor therapy, though current evidence suggests potential benefit for initial response without necessarily extending long-term survival.³⁹ The clinical utility of mutation testing continues to evolve as targeted therapies develop.
Diagnostic Challenges and Practical Solutions
Sample Quality and Technical Considerations
The success of cytological grading depends fundamentally on sample quality, with specific technical requirements that may not be necessary for simple diagnostic confirmation.⁵ Adequate cellularity requiring at least 100 intact mast cells excludes some diagnostic samples from prognostic assessment, emphasizing the need for optimal aspiration techniques.
Needle gauge selection affects sample characteristics, with 25-gauge needles producing less blood contamination but potentially increased cellular trauma, while 22-gauge needles may yield better cellular preservation despite greater blood contamination.⁴⁰ The choice requires balancing competing technical factors based on tumor characteristics and clinical requirements.
Staining method selection significantly impacts both diagnostic accuracy and grading reliability. Wright-Giemsa staining provides superior morphological detail for grading assessment compared to rapid staining methods, though rapid stains may be adequate for diagnostic confirmation.²² The clinical setting and urgency of results may influence staining protocol selection.
Interpretive Challenges in Clinical Practice
Mixed granulation patterns present particular challenges in clinical assessment, requiring determination of the predominant cellular population rather than simple presence or absence of poorly granulated cells.⁴¹ Quantitative approaches may help standardize this assessment and improve consistency across observers.
Poorly granulated MCTs may be confused with other round cell tumors, particularly lymphoma, histiocytic sarcoma, or plasma cell tumors.⁴² The integration of clinical history, imaging findings, and immunohistochemical markers becomes crucial for accurate diagnosis in ambiguous cases.
Reactive changes secondary to inflammation or previous biopsy attempts may mimic malignant features, potentially leading to overgrading and inappropriate treatment escalation.⁴³ The distinction between reactive and neoplastic changes requires experience and correlation with clinical history.
Future Directions and Clinical Applications
Artificial Intelligence and Automated Assessment
The development of automated morphometric analysis systems shows promise for improving reproducibility and accuracy of cytological grading.⁴⁴ Deep learning algorithms have demonstrated superior prognostic value compared to traditional pathologist estimates in preliminary studies, suggesting potential for enhanced diagnostic precision.
Automated nuclear morphometry may particularly benefit assessment of features such as anisokaryosis and pleomorphism, where subjective evaluation creates inter-observer variability.²⁴ The standardization of measurement protocols could improve consistency across different laboratories and practitioners.
The integration of artificial intelligence with traditional morphological assessment may identify subtle morphological features that correlate with biological behavior but remain below the threshold of human recognition, potentially refining prognostic accuracy.⁴⁵
Integration with Molecular Diagnostics
Current research focuses on integrating cytological findings with molecular markers that provide additional prognostic and therapeutic information.⁴⁶ The combination of morphological assessment with KIT mutation analysis, proliferation markers, and other molecular features may enable more precise risk stratification.
Flow cytometric analysis offers opportunities for objective assessment of cellular characteristics and may identify novel prognostic parameters not apparent through traditional morphological evaluation.⁴⁷ The quantitative nature of flow cytometry could complement morphological grading systems.
Clinical Decision-Making Framework
When to Implement Cytological Grading
The decision to pursue cytological grading should consider the clinical context, treatment planning requirements, and available expertise.²⁰ Cases where immediate prognostic information would significantly alter treatment decisions represent the strongest indication for cytological grading assessment.
Patient factors including age, overall health status, and owner treatment preferences may influence the value of cytological grading information. Young, healthy patients with owners committed to aggressive treatment may benefit most from detailed prognostic assessment, while elderly patients or those with significant comorbidities may require different decision-making approaches.
The availability of specialized cytopathology expertise, either in-house or through referral laboratories, may determine the feasibility of implementing cytological grading protocols.⁴⁸ Quality assurance requirements and training needs should be considered in implementation planning.
Integration with Clinical Management
Cytological grading should be integrated with comprehensive clinical assessment rather than used as a standalone prognostic tool.⁴⁹ The combination of anatomical location, clinical presentation, staging results, and cytological grade provides the most complete prognostic picture for treatment planning.
Follow-up protocols should be tailored based on cytological grade, with high-grade tumors requiring intensive monitoring for local recurrence, new tumor development, and metastatic disease.⁵⁰ Low-grade tumors may warrant less intensive surveillance while maintaining vigilance for clinical changes.
The communication of cytological grading results to pet owners requires careful explanation of both the prognostic information and its limitations, ensuring informed consent for treatment decisions while maintaining appropriate hope and realistic expectations.⁵¹
Conclusions and Clinical Recommendations
The cytological assessment of mast cell tumors has evolved from simple diagnostic confirmation to sophisticated prognostic evaluation that can guide clinical decision-making from initial diagnosis through long-term management.⁵'²⁰ The development of validated cytological grading systems represents a significant advance in veterinary oncology, providing clinicians with immediate prognostic information that was previously available only through histopathological assessment.
However, the clinical implementation of cytological grading requires careful consideration of technical requirements, quality assurance protocols, and integration with comprehensive clinical assessment.⁵² The system works best when applied by experienced cytopathologists or clinicians with specialized training, supported by appropriate quality control measures and correlation with clinical outcomes.
The most practical approach to cytological grading involves selective implementation based on clinical need, with emphasis on cases where immediate prognostic information would significantly impact treatment decisions.²⁰ The integration of cytological grading with anatomical considerations, clinical staging, and ancillary diagnostic methods provides the most comprehensive prognostic assessment for optimal patient management.
Future developments in automated analysis, molecular integration, and species-specific validation promise continued improvement in diagnostic accuracy and clinical utility.⁴⁴'⁴⁶ The success of cytological grading systems for canine MCTs provides a model for similar approaches in other tumor types and species, highlighting the continued evolution and expanding role of cytopathology in veterinary medicine.
The practical implementation of cytological grading should focus on quality over quantity, emphasizing accurate assessment of suitable cases rather than attempting to grade every diagnostic sample. This approach ensures optimal clinical utility while maintaining the high standards necessary for reliable prognostic assessment that can guide critical treatment decisions for our patients.
References
Villamil JA, Henry CJ, Bryan JN, et al. Identification of the most common cutaneous neoplasms in dogs and evaluation of breed and age distributions for selected neoplasms. J Am Vet Med Assoc. 2011;239(7):960-965.
Sledge DG, Webster J, Kiupel M. Canine cutaneous mast cell tumors: A combined clinical and pathologic approach to diagnosis, prognosis, and treatment selection. Vet Clin North Am Small Anim Pract. 2016;46(4):631-655.
Duncan JR, Prasse KW. Cytology of canine cutaneous round cell tumors. Vet Pathol. 1979;16(6):673-679.
Macy DW. Canine and feline mast cell tumors: biologic behavior, diagnosis, and therapy. Semin Vet Med Surg Small Anim. 1986;1(1):72-83.
Camus MS, Priest HL, Koehler JW, et al. Cytologic Criteria for Mast Cell Tumor Grading in Dogs With Evaluation of Clinical Outcome. Vet Pathol. 2016;53(6):1117-1123.
London CA, Seguin B. Mast cell tumors in the dog. Vet Clin North Am Small Anim Pract. 2003;33(3):473-489.
Murphy S, Sparkes AH, Brearley MJ, et al. Relationships between the grade of cutaneous mast cell tumours in dogs, their survival and the efficacy of surgical resection. Vet Rec. 2004;154(24):743-746.
Berlato D, Bulman-Fleming J, Clifford CA, et al. Value, Limitations, and Recommendations for Grading of Canine Cutaneous Mast Cell Tumors: A Consensus of the Oncology-Pathology Working Group. Vet Pathol. 2021;58(5):858-863.
Hillman LA, Garrett LD, de Lorimier LP, et al. Biological behavior of oral and perioral mast cell tumors in dogs: 44 cases (1996-2006). J Am Vet Med Assoc. 2010;237(8):936-942.
Kiupel M, Webster JD, Miller RA, Kaneene JB. Impact of tumour depth, tumour location and multiple synchronous masses on the prognosis of canine cutaneous mast cell tumours. J Vet Med A Physiol Pathol Clin Med. 2005;52(6):280-286.
Thompson JJ, Pearl DL, Yager JA, et al. Canine subcutaneous mast cell tumor: characterization and prognostic indices. Vet Pathol. 2011;48(1):156-168.
Sabattini S, Giantin M, Vascellari M, et al. Grading Cutaneous Mast Cell Tumors in Cats. Vet Pathol. 2019;56(1):43-49.
Molander-McCrary H, Henry CJ, Potter K, et al. Cutaneous mast cell tumors in cats: 32 cases (1991-1994). J Am Anim Hosp Assoc. 1998;34(4):281-284.
Lepri E, Riccardi E, Dall'Aglio C, et al. Clinical features, treatment and outcome of intestinal mast cell tumours in cats: 11 cases. J Feline Med Surg. 2015;17(12):1023-1029.
Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet Pathol. 1984;21(5):469-474.
Northrup NC, Harmon BG, Gieger TL, et al. Variation among pathologists in histologic grading of canine cutaneous mast cell tumors. J Vet Diagn Invest. 2005;17(3):245-248.
Northrup NC, Howerth EW, Harmon BG, et al. Variation among pathologists in the histologic grading of canine cutaneous mast cell tumors with uniform use of a single grading reference. J Vet Diagn Invest. 2005;17(6):561-564.
Stefanello D, Buracco P, Sabattini S, et al. Comparison of 2- and 3-category histologic grading systems for predicting the presence of metastasis at the time of initial evaluation in dogs with cutaneous mast cell tumors: 386 cases (2009-2014). J Am Vet Med Assoc. 2015;246(7):765-769.
Kiupel M, Webster JD, Bailey KL, et al. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet Pathol. 2011;48(1):147-155.
Berlato D, Bulman-Fleming J, Clifford CA, et al. Value, Limitations, and Recommendations for Grading of Canine Cutaneous Mast Cell Tumors: A Consensus of the Oncology-Pathology Working Group. Vet Pathol. 2021;58(5):858-863.
Hergt F, von Bomhard W, Kent MS, Hirschberger J. Use of a 2-tier histologic grading system for canine cutaneous mast cell tumors on cytology specimens. Vet Clin Pathol. 2016;45(3):477-483.
Sabattini S, Renzi A, Marconato L, et al. Comparison between May-Grünwald-Giemsa and rapid cytological stains in fine-needle aspirates of canine mast cell tumor: diagnostic and prognostic implications. Vet Comp Oncol. 2018;16(4):511-517.
Scarpa F, Sabattini S, Bettini G. Cytological grading of canine cutaneous mast cell tumours. Vet Comp Oncol. 2016;14(3):245-251.
Haghofer A, Parlak E, Bartel A, et al. Nuclear pleomorphism in canine cutaneous mast cell tumors: Comparison of reproducibility and prognostic relevance between estimates, manual morphometry, and algorithmic morphometry. Vet Pathol. 2025;62(2):161-177.
Kiupel M, Camus M. Diagnosis and Prognosis of Canine Cutaneous Mast Cell Tumors. Vet Clin North Am Small Anim Pract. 2019;49(5):819-836.
Paes PRO, Horta RS, Luza LC, et al. Inclusion of fibroblasts and collagen fibrils in the cytologic grading of canine cutaneous mast cell tumors. Vet Clin Pathol. 2022;51(3):339-348.
Owen LN. TNM classification of tumours in domestic animals. World Health Organization. Geneva; 1980.
Thamm DH, Vail DM. Mast cell tumors. In: Withrow SJ, Vail DM, Page RL, eds. Withrow and MacEwen's Small Animal Clinical Oncology. 5th ed. St. Louis: Elsevier Saunders; 2013:335-355.
Weishaar KM, Thamm DH, Worley DR, Kamstock DA. Correlation of nodal mast cells with clinical outcome in dogs with mast cell tumour and a proposed classification system for the evaluation of node metastasis. J Comp Pathol. 2014;151(4):329-338.
Book AP, Fidel J, Wills T, et al. Correlation of ultrasound findings, fine-needle aspiration cytology, and histopathology for assessment of lymph node metastasis in dogs with mast cell tumors: 32 cases (2005-2007). J Am Vet Med Assoc. 2011;238(11):1394-1401.
Schultheiss PC, Gardiner DW, Rao S, et al. Association of histologic tumor characteristics and size of surgical margins with clinical outcome after surgical removal of cutaneous mast cell tumors in dogs. J Am Vet Med Assoc. 2011;238(11):1464-1469.
Hume CT, Kiupel M, Rigatti L, et al. Outcomes of dogs with grade 3 mast cell tumors: 43 cases (1997-2007). J Am Anim Hosp Assoc. 2011;47(1):37-44.
Vascellari M, Giantin M, Capello K, et al. Expression of Ki67, BCL-2, and COX-2 in canine cutaneous mast cell tumors: association with grading and prognosis. Vet Pathol. 2013;50(1):110-121.
Marouda C, Anagnostou T, Brunetti B, et al. Cutaneous Canine Mast Cell Tumor: The Use of Proliferative Markers (Ki-67 and Ki-67 × AgNOR) in Cytological Samples for Diagnosis and Prognosis. Vet Sci. 2024;11(1):23.
Giantin M, Vascellari M, Morello EM, et al. c-KIT messenger RNA and protein expression and mutations in canine cutaneous mast cell tumors: correlations with post-surgical prognosis. J Vet Diagn Invest. 2012;24(1):116-126.
Reguera MJ, Ferrer L, Rabanal RM, et al. Canine mast cell tumors express stem cell factor receptor. Am J Dermatopathol. 2000;22(1):49-54.
Kiupel M, Webster JD, Kaneene JB, et al. The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Vet Pathol. 2004;41(4):371-377.
Webster JD, Kiupel M, Kaneene JB, et al. The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Vet Pathol. 2004;41(4):371-377.
London CA, Malpas PB, Wood-Follis SL, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15(11):3856-3865.
Berzina I, Sharkey LC, Matise I, et al. Correlation between cytologic and histopathologic diagnoses of bone lesions in dogs: 47 cases (2005-2006). J Am Vet Med Assoc. 2008;233(8):1258-1263.
Sabattini S, Scarpa F, Berlato D, Bettini G. Histologic grading of canine mast cell tumor: is 2 better than 3? Vet Pathol. 2015;52(1):70-73.
Cowell RL, Tyler RD, Meinkoth JH, DeNicola DB. Diagnostic Cytology and Hematology of the Dog and Cat. 3rd ed. St. Louis: Mosby Elsevier; 2008.
Raskin RE, Meyer DJ. Canine and Feline Cytology: A Color Atlas and Interpretation Guide. 3rd ed. St. Louis: Elsevier Saunders; 2016.
Bertram CA, Aubreville M, Marzahl C, et al. A large-scale dataset for the development of automated deep learning-based algorithms for dermatological diagnosis in dogs and cats. Sci Data. 2019;6:147.
Aubreville M, Bertram C, Klopfleisch R, Maier A. SlideRunner: A Tool for Massively Annotating Whole Slide Images. In: Bildverarbeitung für die Medizin 2018. Springer Vieweg; 2018:309-314.
Tamlin VS, Bottema CDK, Peaston AE. Comparative aspects of mast cell neoplasia in animals and the role of KIT in prognosis and treatment. Vet Med Sci. 2020;6(1):3-18.
Sledge DG, Patrick DJ, Fitzgerald SD, et al. Differences in expression of urokinase plasminogen activator and plasminogen activator inhibitor-1 in canine oral, cutaneous, and systemic histiocytic sarcomas. Vet Pathol. 2006;43(6):871-882.
Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames: Iowa State University Press; 2016.
Morris JS, Dobson JM. Small Animal Oncology. Oxford: Blackwell Science; 2001.
Berlato D, Murphy S, Monti P, et al. Clinical outcomes of dogs with high-grade cutaneous mast cell tumors. Front Vet Sci. 2024;11:1519636.
Henry CJ, Higginbotham ML. Cancer Management in Small Animal Practice. Maryland Heights: Saunders Elsevier; 2010.
Withrow SJ, Vail DM, Page RL, editors. Withrow and MacEwen's Small Animal Clinical Oncology. 5th ed. St. Louis: Elsevier Saunders; 2013.