Cytologic Evaluation of Lymphoma: Grading Challenges, Ancillary Testing, and AI Applications
Introduction
Lymphoma represents one of the most common hematopoietic neoplasms in veterinary medicine, affecting both dogs and cats with varying clinical presentations and biological behaviors. While cytopathology serves as a fundamental aid in identifying lymphoma and can be used as a screening test to predict grade and phenotype, accuracy decreases significantly when further characterization is needed. This review examines the cytologic differences between low-grade and high-grade lymphoma, discusses diagnostic challenges, and explores the role of ancillary testing and artificial intelligence when traditional cytologic methods prove insufficient.
Cytologic Differences Between Low-Grade and High-Grade Lymphoma
High-Grade Lymphoma
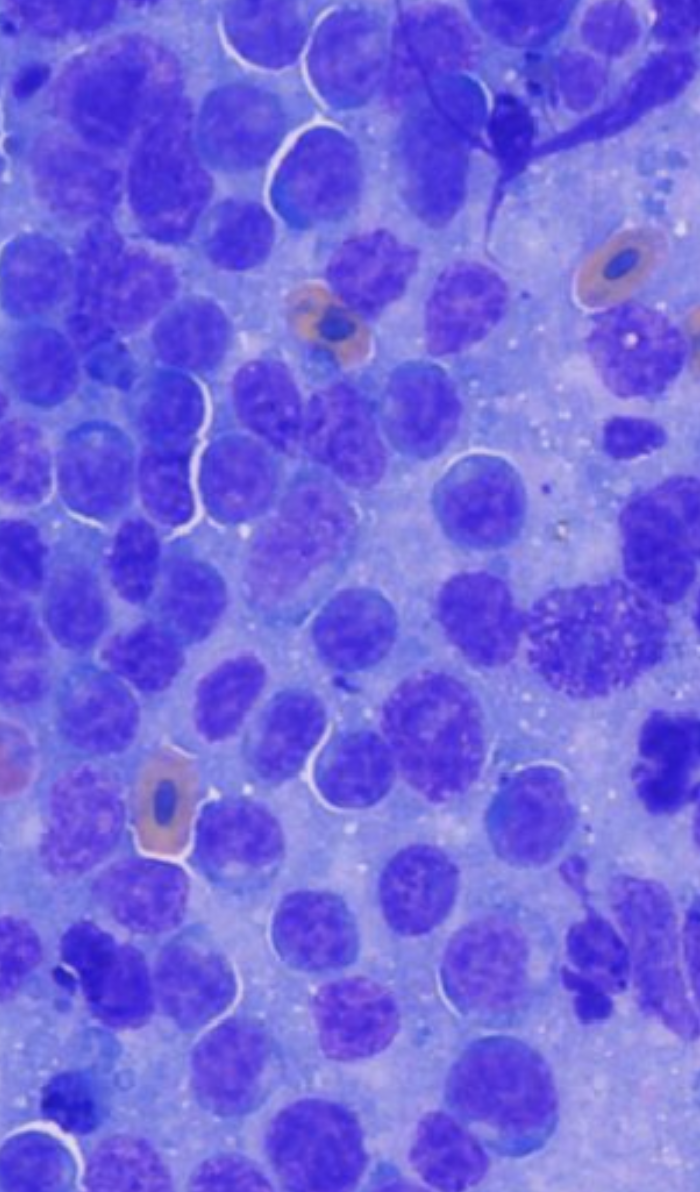
High-grade lymphomas are characterized by populations of large, immature lymphocytes that exhibit significant morphologic atypia. Unlike most malignant tumors, lymphoma is commonly characterized by a uniform population of cells that are larger than normal lymphoid cells, and variation in morphology is not necessary for a diagnosis of malignancy with this tissue type (Valli et al., 2011). Key cytologic features include:
Nuclear size: Large nuclei, typically 2-3 times the diameter of a red blood cell
Nuclear morphology: Vesicular chromatin pattern with prominent, often multiple nucleoli
Mitotic activity: High mitotic index with frequent abnormal mitotic figures
Cell uniformity: Monomorphic population of neoplastic cells
Cytoplasmic features: Moderate to abundant basophilic cytoplasm
Lymphoblastic lymphoma (LBL) cells are characterized by a dispersed chromatin pattern that obscures nucleoli in cells with nuclei of intermediate size (Valli et al., 2011). These high-grade lymphomas typically present as diffuse effacement of lymph node architecture and are readily identified on fine needle aspiration cytology (Durham et al., 2016).
Low-Grade Lymphoma
Low-grade lymphomas present significantly more diagnostic challenges cytologically. Well-differentiated, mature, small lymphocytes are the hallmark of low-grade intestinal T-cell lymphoma (LGITL) in cats (Kiupel et al., 2023). Characteristic features include:
Nuclear size: Small to intermediate-sized nuclei, often similar to reactive lymphocytes
Nuclear morphology: Condensed chromatin with inconspicuous nucleoli
Cell uniformity: Less obvious monomorphism, requiring careful evaluation
Mitotic activity: Low mitotic index
The primary challenge with low-grade lymphomas is distinguishing them from reactive lymphoid hyperplasia. If mostly medium to large cells are present, lymphoma is a possibility. However, if there is marked variation and small lymphocytes are present, that is more typical of hyperplasia.
Challenges in Cytologic Evaluation
Sampling Limitations
Cytologic evaluation has limitations that can hinder a definitive diagnosis of lymphoma (Skeldon & Dewhurst, 2020). These include:
Sampling of a lymph node that is not fully effaced by neoplastic lymphocytes
Disproportional sampling of a large reactive lymphoid nodule
Lymphomas comprised of predominately small lymphocytes
Prior administration of glucocorticoids reducing neoplastic lymphocyte numbers
Misinterpretation by the evaluator
Smear preparations lacking intact and/or well-spread cells (Martini et al., 2022)
Inter-observer Variability and Diagnostic Performance
A comprehensive multi-center study by Martini et al. (2022) examining the performance of lymph node cytopathology in dogs provided crucial insights into the limitations of cytologic diagnosis. This landmark study involved 161 lymph node samples from 139 dogs with enlarged peripheral lymph nodes, evaluated by six experienced examiners who independently provided interpretations based solely on cytopathology.
The study revealed that while cytopathology was accurate and precise in identifying lymphoma samples (sensitivity = 93.1%, specificity = 89.5%, with kappa inter-observer agreement = 0.79), accuracy dropped significantly when further characterization was needed. When examiners attempted to classify lymphomas by grade and phenotype, the accuracy decreased substantially, with sensitivity ranging from 60-80% depending on the specific category. All examiners claimed that they were more confident in predicting grade than phenotype, highlighting the inherent challenges in immunophenotype determination from cytology alone.
Most significantly, when attempting to assign specific World Health Organization (WHO) histopathological subtypes based on cytology alone, the accuracy fell to approximately 40-60%. This does not reflect a failure of cytology as a diagnostic tool, but rather the inherent limitations of morphology alone in definitively subclassifying lymphomas. The study concluded that cytopathology represents a fundamental aid in identifying lymphoma and can be used as a screening test to predict grade and phenotype, with complementary ancillary techniques recommended to confirm these findings and provide definitive classification.
Small Cell Lymphomas
Small cell lymphomas present particular diagnostic challenges. The samples are small and may not be able to determine between inflammation and cancer, and the chance of obtaining a certain diagnosis is lower with cytology than with biopsy (NC State Veterinary Hospital, 2024). This is especially problematic in feline cases where cytologic examination of fine needle aspirates from the intestinal wall is not helpful for reaching a definitive diagnosis of either lymphoplasmacytic enteritis or low-grade intestinal T-cell lymphoma due to the lack of architectural information and overlapping cellular morphology (Kiupel et al., 2023).
Ancillary Testing to Complement Cytologic Evaluation
When cytologic findings suggest lymphoma but the disease process lacks clear morphologic features that allow for confident diagnosis and classification, complementary testing is recommended to confirm the diagnosis and provide prognostic information. These ancillary tests are particularly valuable when:
Small cell lymphomas are cytologically indistinguishable from reactive lymphoid hyperplasia
Immunophenotype cannot be reliably determined from morphology alone
Definitive WHO subtype classification is needed for prognostication
Monoclonality needs to be confirmed in ambiguous cases
Immunophenotyping
Immunohistochemistry (IHC) is essential for determining B-cell versus T-cell lineage (Valente et al., 2024). Canine lymphomas of T-cell origin tend to have worse prognoses than those of B-cell origin, with canine T-zone lymphoma being a notable exception with an indolent clinical course and survival times often of two to four years (Skeldon & Dewhurst, 2020). Standard markers include CD3 (T-cell) and PAX5/CD79a (B-cell) (Sampaio et al., 2023).
Flow Cytometry allows identification of cell-associated proteins that distinguish neoplastic cell populations by lineage and aberrant protein expression, allowing more precise classification for better prognostication and tailored therapy (VDx Veterinary Diagnostics, 2024). Flow cytometry has advantages over traditional immunohistochemistry in that multiple cell proteins can be assessed simultaneously, sample collection is minimally invasive, and results are rapidly available. However, the limited number of antibodies available in veterinary medicine compared with human medicine, and the risk of misdiagnosing different subtypes of lymphoma, such as indolent lymphomas, still represent major disadvantages (Aresu et al., 2016).
PCR for Antigen Receptor Rearrangement (PARR)
PARR is particularly useful for determining whether cancer is present by identifying whether the lymphocytes are all basically the same cell (and therefore cancerous), or whether they are very mixed with no particularly prominent population, and therefore reactive (Marsh, 2024). In a recent study, PARR revealed monoclonal rearrangement/clonality in 33 cases, with 84.85% of these being B-cell and 15.15% T-cell lymphomas (Valente et al., 2024). PARR is particularly useful for distinguishing reactive hyperplasia from neoplasia in ambiguous cases, though it can have limitations as some cancer cells have receptors so mutated that the PARR analysis can't find them, leading to false negatives (Marsh, 2024).
Cell Block Preparation
Cell block analysis identified lymphoma in 30 dogs and suggested lymphoma or a round cell neoplasm in 8 cases, with cell block immunocytochemistry confirming lymphoma in 35 dogs, comprising 80% B-cell and 20% T-cell lymphomas (Valente et al., 2024). This technique allows for application of immunohistochemical stains on cytologic material and provides better morphologic detail than conventional smears. However, immunocytochemistry on cytological smears yielded inconclusive results in 50% of cases in one study (Valente et al., 2024).
Genomic Analysis
Genomic tumor analysis can provide clinical guidance for diagnostically challenging cancers, with a clinical utility of 86% in providing diagnostic clarity, prognostic information, and/or therapeutic options (Phillips et al., 2023). The genomic assay was diagnostically supportive of either a broad histologic class or specific histology in 45% of patients, and was able to differentiate malignant versus likely benign neoplasia in 9% of patients.
The Role of Artificial Intelligence in Lymphoma Diagnosis
Artificial intelligence (AI) is emerging as a transformative tool in veterinary pathology, with particular promise for lymphoma diagnosis and classification.
Current AI Applications in Cytology and Histology
Moichor is leading the advancement in this area, and Pathview users can look forward to this new feature.
AI-based workflows for the classification of lymphoma with regards to nuclear size (small, intermediate, and large) have been developed using deep learning models trained on histological images of canine lymphoma with individually labeled nuclei (Haghofer et al., 2023). Chu et al. developed an AI model to identify small, intermediate, and large lymphocytes in photomicrographs of canine lymph node aspirates, which was then used to diagnose lymphoma with an accuracy of 97.14% (Neal, 2024).
GoogLeNet transfer learning has been used to classify three classes of canine lymphoma from whole slide images, achieving 99% accuracy in the test set (Xiao et al., 2025). Current research at Virginia Tech, led by Ph.D. student Christina Pacholec, aims to harness artificial intelligence to analyze cytological images for early detection of lymphoma through less invasive, quicker, and more cost-effective methods (Virginia Tech News, 2024). The project's initial phase focuses on training an AI tool to distinguish between lymphoma-affected and healthy dogs by analyzing cytology images.
Future Potential and Implementation
AI holds the promise of redefining diagnostic precision and therapeutic outcomes across veterinary oncology, whether through automated analysis of medical images or integration of multi-omics data for personalized treatment planning (Pinard, 2025). AI has transformative potential in veterinary pathology in tasks ranging from cell enumeration and cancer detection to prognosis forecasting, virtual staining techniques, and individually tailored treatment plans (Pacholec et al., 2024). Machine learning models have also been developed to predict likelihood of in vivo chemotherapy response in canine lymphoma using ex vivo drug sensitivity and immunophenotyping data (Bohannan et al., 2021).
While AI shows tremendous promise, the future of veterinary pathology must balance harnessing AI's potential while intentionally mitigating its risks, ensuring the welfare of animals and the integrity of the veterinary profession are safeguarded (Pacholec et al., 2024). Current limitations include the need for large, high-quality datasets and the requirement for rigorous validation before clinical implementation. Despite adoption in veterinary hematology and urinalysis, AI-driven image analysis implementation has been relatively slow in veterinary cytopathology, especially compared to other imaging-heavy disciplines such as radiology and histopathology (Neal, 2024).
Clinical Pathologist's Perspective
From the standpoint of a veterinary clinical pathologist, several key principles should guide the diagnostic approach to lymphoma:
Cytology as a Screening Tool: Cytology excels at identifying lymphoma (93.1% sensitivity) and provides a valuable initial assessment of grade and phenotype. When morphologic features do not permit confident diagnosis or classification, complementary testing should be pursued rather than viewing cytology as inadequate.
Ancillary Testing is Complementary: In cases where cytologic morphology is ambiguous or subclassification is needed, the combination of immunophenotyping, PARR, and other molecular techniques significantly improves diagnostic confidence and provides essential prognostic information.
AI Integration: AI-based tools show remarkable accuracy (97-99%) in lymphoma classification and represent a valuable adjunct to traditional cytologic evaluation.
Quality Assurance: Standardization of techniques and ongoing education are crucial for maintaining diagnostic accuracy across laboratories.
Conclusion
The diagnosis and grading of lymphoma through cytologic evaluation continues to evolve with advancing technology and improved understanding of tumor biology. Cytologic examination remains a highly valuable diagnostic tool with excellent sensitivity (93.1%) for identifying lymphoma. When the disease process lacks clear morphologic features for confident diagnosis or classification, complementary ancillary testing provides essential confirmation and prognostic information. The emergence of AI technologies promises to enhance diagnostic accuracy and efficiency, with current models achieving 97-99% accuracy in lymphoma classification. As the field advances, the collaborative approach between clinical pathologists, oncologists, and technology developers, combined with rigorous validation of AI tools, will be crucial in providing the most accurate diagnoses and best outcomes for our patients.
References
Aresu, L., Martini, V., Rossi, F., et al. (2016). Canine indolent and aggressive lymphoma: clinical spectrum with histologic correlation. Frontiers in Veterinary Science, 3, 77.
Bohannan, Z., Pudupakam, R.S., Koo, J., et al. (2021). Predicting likelihood of in vivo chemotherapy response in canine lymphoma using ex vivo drug sensitivity and immunophenotyping data in a machine learning model. Veterinary and Comparative Oncology, 19, 160-71.
Durham, A.C., Selting, K.A., Rout, E.D., et al. (2016). The comparative diagnostic features of canine and human lymphoma. Veterinary Sciences, 3(2), 11.
Haghofer, A., Fuchs-Baumgartinger, A., Lipnik, K., et al. (2023). Histological classification of canine and feline lymphoma using a modular approach based on deep learning and advanced image processing. Scientific Reports, 13, 19436.
Kiupel, M., Smedley, R., Pfent, C., et al. (2023). ACVIM consensus statement guidelines on diagnosing and distinguishing low-grade neoplastic from inflammatory lymphocytic chronic enteropathies in cats. Journal of Veterinary Internal Medicine, 37, 794-816.
Marsh, N. (2024). Lymphocytes, part 4: further tests (PARRty time). Veterinary Times. Available at: https://www.vettimes.com/news/vets/opinion/lymphocytes-part-4-further-tests-parrty-time
Martini, V., Marano, G., Aresu, L., et al. (2022). Performance of lymph node cytopathology in diagnosis and characterization of lymphoma in dogs. Journal of Veterinary Internal Medicine, 36(1), 204-214.
NC State Veterinary Hospital. (2024). Medical Oncology: Feline Low-Grade Lymphoma. Available at: https://hospital.cvm.ncsu.edu/services/small-animals/cancer-oncology/oncology/feline-low-grade-lymphoma/
Neal, S. (2024). Artificial intelligence in veterinary clinical pathology—An introduction and review. Veterinary Clinical Pathology, Early view. doi:10.1111/vcp.70012
Pacholec, C., Flatland, B., Xie, H., & Zimmerman, K. (2024). Harnessing artificial intelligence for enhanced veterinary diagnostics: A look to quality assurance, Part I Model development. Veterinary Clinical Pathology, Early view.
Phillips, J.C., Linden, M.A., Reinero, C., et al. (2023). Genomic tumor analysis provides clinical guidance for the management of diagnostically challenging cancers in dogs. Journal of the American Veterinary Medical Association, 261(5), 489-499.
Pinard, C.J. (2025). The future is here: an introduction to the Veterinary Oncology collection on Artificial Intelligence and Informatics. Veterinary Oncology, 2, 10.
Sampaio, F., Marrinhas, C., Fonte-Oliveira, L., et al. (2023). Detection of lymphoid markers (CD3 and PAX5) for immunophenotyping in dogs and cats: Comparison of stained cytology slides and matched cell blocks. Veterinary Sciences, 10(2), 157.
Skeldon, N., & Dewhurst, E. (2020). The perceived and actual diagnostic utility of veterinary cytologic samples. Journal of Small Animal Practice, 50(4), 180-185.
Valente, P.C.L.G., Santos, M., Oliveira, P.N., et al. (2024). Comparison of the accuracy of minimally invasive techniques (cytology, cell block, immunocytochemistry and clonality assay) in the diagnosis of canine multicentric lymphoma. Research in Veterinary Science, 176, 105320.
Valli, V.E., San Myint, M., Barthel, A., et al. (2011). Classification of canine malignant lymphomas according to the World Health Organization criteria. Veterinary Pathology, 48(1), 198-211.
VDx Veterinary Diagnostics. (2024). Flow Cytometry. Available at: https://vdxpathology.com/FlowCytometry.htm
Virginia Tech News. (2024). New research aims to use AI to make cancer diagnostics for pets more available and affordable. Available at: https://news.vt.edu/articles/2024/03/vetmed-AI-cancer-diagnostics.html
Xiao, S., Dhand, N.K., Wang, Z., et al. (2025). Review of applications of deep learning in veterinary diagnostics and animal health. Frontiers in Veterinary Science, 12, 1511522.